Scientists at the Swiss Higher Technical School of Zurich (Eidgenössische Technische Hochschule Zürich, ETH Zürich) by means of genetic reprogramming of stem cell adipose tissue obtained beta cells that are virtually identical to natural. This study may be one of the most important steps in the personalized treatment of diabetes. The results of the experiments were published on April 11, 2016 by Nature Communications.
A team led by Martin Fussenegger, professor of biotechnology, and the Biosystems Science and Technology bioengineering department at ETH Zurich in Basel, conducted an experiment that to date has not been matched by anyone. The experts removed stem cells from the adipose tissue of a 50-year-old experimental patient and reprogrammed them to differentiate into functional beta cells.
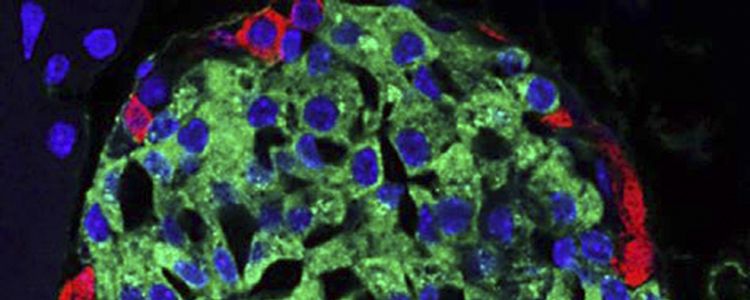
In the presence of glucose, the beta cells resulting from an artificially created “genetic program”, produce the hormone insulin as well as the natural cells of the pancreas.
Researchers from Basel altered the stem cells through complex genetic circuits via artificially created – “genetic software”, which was designed to play a role in key growth factors involved in the maturation of cells.
A key role in this process is played by the growth factors Ngn3, Pdx1 and MAFA. The concentration of these factors changed throughout the process of differentiation.
For example, MAFA was absent at the beginning of the process, and appeared only on the fourth day and the final stage, when its concentration increased sharply and remained high.
Changes in the concentration of Ngn3 and Pdx1 followed a rather complex pattern: while Ngn3 concentration increases and then decreases, the level of Pdx1 increases in both early and late maturing.
Fussenegger emphasized it was crucial to attempt the reproduction of natural processes as closely as possible to produce functional beta cells. “Synchronization time and the number of these growth factors is important.”
Fussenegger believes a breakthrough has been made in creating an artificial genetic circuit that has been successfully used in the genetic reprogramming of beta cells. To date, scientists controlled the process of differentiation of stem cells by adding various external chemicals and proteins in the laboratory.
“The challenge is not only to add the required number of components at the right time, but also due to the ineffectiveness of this method, it is impossible to play on a large scale,” Fussenegger said. In contrast, this new method was able to transform the beta cells from three of the stem cells taken from adipose tissue.
The resulting beta cells are not only very similar to their natural counterparts but both species contain secretory granules that store insulin. Artificial beta cells are also functionally very similar to the natural.
“They are now producing not as much insulin as natural beta-cells,” Fussenegger admitted. However, the main achievement of the scientists is they were the first to manage to reproduce the entire sequence of the natural process of differentiation of stem cells in the beta cells.
In the future this new technique may allow Swiss researchers to transplant new functional beta cells in patients with diabetes from their own adipose tissue.
Preliminary transplant beta cells demand the suppression of the immune system of the recipient, as with any transplantation of donated organs or tissue.